This IS The Cure For Cancer
Growing Intrigue Surrounding GcMAF
Cancer Treatment
Written By Bill Sardi
Published: May 2017
GcMAF (Gc-protein macrophage activating factor) is back in the news again.1 GcMAF gained worldwide attention in 2008 when this health writer broke the story about its ignored cancer-killing properties. It is the body’s way of curing cancer without side effects, deliberately concealed for 26 years.
To reignite a growing controversy, more recently BBC News issued a scathing report against GcMAF. An undercover BBC investigation found a health shop in Bournemouth, UK was still selling this unlicensed medicine after it had been banned. (But this would be like banning red blood cells. GcMAF is a blood fraction.) The BBC report then prompted Cancer Research UK to say there “is no scientific evidence to show that GcMAF works as a treatment for cancer2 and “[i]t is an injectable blood product, the source of which we have no knowledge.”3
A GcMAF biomedical scientist (Lynda Thyer) immediately responded to the BBC News report in a letter published in the Bournemouth Echo saying there are over 300 scientific research papers, peer reviewed and published in major scientific journals, 11 of them with her name on them. In fact, GcMAF has 20 beneficial effects in the body. GcMAF is naturally made in the body by removal of two sugars from Gc-Protein in blood. This process can be replicated in the laboratory to produce billionths of a gram of GcMAF, which is all you need.
The science behind GcMAF is far from obscure. In fact, the U.S. Department of Energy, Office of Science, itself posts abstracts on GcMAF to over 100 published papers.4
Cancer Research UK said their initial interest in GcMAF was piqued by an article written by my co-author Timothy Hubbell and me in 2008 entitled “Cancer Cured for Good,” which was also published in Health Freedom News.5 That report called attention to four published studies by researcher Nobuto Yamamoto where GcMAF was successfully used to quell human breast, colon, lung, and prostate cancer.
The main point of that report was that cancer researchers were not devoting any attention to GcMAF. One would think the National Cancer Institute would be calling for an immediate scientific conference on the topic to redirect research funds to this promising cancer therapy.
Also, not a single oncologist contacted this author after I wrote about GcMAF in 2008. I found that very strange.
Furthermore, the U.S. Department of Defense actually funded a study of GcMAF, which “shows strong inhibitory activity of GcMAF on prostate tumor cells independent of its macrophage activation.”6 Cancer Research UK is not oblivious to this published science, because for years they’ve had a webpage denigrating GcMAF.
The GcMAF Genie Has Escaped From the Bottle
Despite efforts to quell efforts to commercialize GcMAF as a therapeutic agent for cancer, it has escaped from an attempted stranglehold by health authorities.
A developmental drug company in Israel is proceeding ahead with preliminary human safety studies using GcMAF. The company, EFRANAT, provides a convincing visual picture of the promise posed by GcMAF in photographs of a dog that experienced complete disease-free recovery from T-cell cutaneous lymphoma with GcMAF therapy. The dog experienced a complete curative effect after only three injectable doses of GcMAF (see below).7
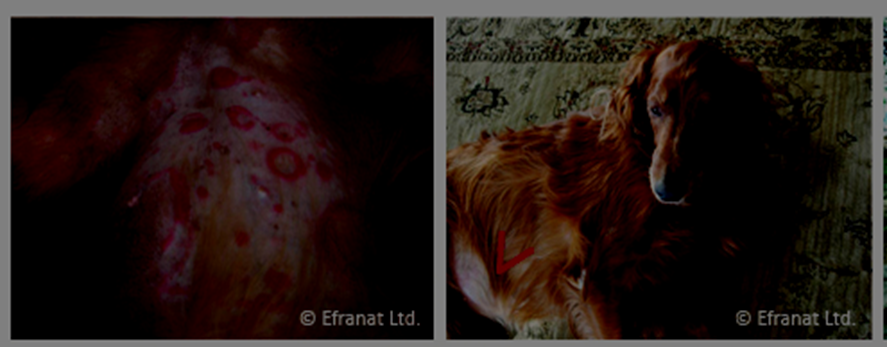
Summary of results: 2 of 14 dogs experienced complete recovery; 7 of 14 dogs experienced shrinkage of their tumors and their cancer stabilized and did not spread; 5 of 14 dogs did not respond to treatment.
Researchers in Japan have opened numerous clinics where GcMAF therapy is being offered on a broad scale.8 The Saisei Mirai Clinics in Japan actively report on their results with GcMAF in published papers.9 By March of 2013, some 345 patients had been treated with GcMAF in Japan.10
Immuno Biotech researchers say Saisei Mirai’s product is not concentrated GcMAF but is blood serum with just the red blood cells removed. Perhaps that is why in the above paper, they only report 3 out of 345 successes. But, nonetheless, efforts to commercialize GcMAF are broadening.
Target of Attack
Sustained attacks by regulatory agencies against GcMAF therapy have been directed at Immuno Biotech, the sanctioned company that braved to produce GcMAF and make it available to cancer patients, and was based on the isle of Guernsey (in the English Channel off the coast of Normandy). David Noakes is the founder and CEO of Immuno Biotech and still manages a transparent operation of his company.
The company states that 300 scientists in eight nations have published research papers on GcMAF in the past 24 years and Immuno Biotech has employed scientists who have written 33 of those research papers. (See GcMAF.eu) Here is a link to one of those published research studies.11
Immuno Biotech developed a 22-step extraction process to yield this blood fraction from human plasma. The company’s products, GcMAF and GOLEIC – the latter a combination of olive oil, Vitamin D, and GcMAF – also undergo nine batch tests for sterility and potency and are then administered by needle injection. Its scientists claim they had developed a technology to produce a stabilized, orally consumed version of GcMAF just as they were closed down by public health authorities.
Immuno Biotech has experience in treating over 11,500 patients with GcMAF, approximately 2000 of them being cancer patients, without side effects. The company’s time-lapse videos of live cancer cells being destroyed by GOLEIC can be viewed online.12
Yet, despite having demonstrated complete cures for cancer, a fact recorded by BBC News cameras that were never aired on television, David Noakes has been thrown into jail four times, his bank accounts and other assets have been seized, his staff has been terrorized and one of them even thrown into French prison without charges. Still, he persists in doing the right thing.
Compelling Patient Reports
The GcMAF story has escaped attempted censorship. There are compelling reports from patients that have been aired in public and are a bit disarming to skeptics.
- First, there is this link to a newspaper account of remission from a rare immune system disorder.13
- The Daily Star in the UK published a first-person report on April 17, 2016 of a woman with cancer who says GcMAF saved her life.14
- Then there is the video testimony of a terminal cancer patient who reports she was cured and is healthy three years following GcMAF treatment.15
- Plus, the heartfelt video testimony of Teri Davis Newman at https://www.youtube.com/watch?v=fkJ2lq0odQI.
Cancer Research UK (CRUK) must have their blinders on. None are so blind as those who will not see. GcMAF even remedies that problem too via inhibition of abnormal blood vessels that can result in blindness.16 Perhaps the fact CRUK have Big Pharma directors on their board, and direct everyone to the poison of chemotherapy, has something to do with this.
Duty to Warn
But good science isn’t chasing away the critics. The chief pharmacist on the isle of Guernsey says he has a public duty to warn people of serious risk posed by GcMAF.17 But in 11,500 patients, none have reported side effects, unlike chemotherapy, which kills half of the terminal patients it treats. To the contrary, the chief pharmacist has scared patients away from the only treatment that might benefit them.
Set up for Public Ridicule
David Noakes, Immuno Biotech’s founder, was even set up for personal ridicule in a locally publicized charge of sex discrimination and had to pay a £10,000 fine. The truth, however, was far different than depicted in the local press, as Noakes was supported by his many female employees. Moreover, the applicant appears to serially sue employers for a living.
At the same time, hundreds of Guernsey citizens are reported to have pleaded with health officials to halt the ban on GcMAF there.18 Blocked by the heartless authorities from receiving GcMAF, twelve of the patients, who had been recovering from cancer, then died of it.
Ireland Issuing a Warning
Fearing a widespread break of cancer patients from their oncologists who perform chemotherapy that is only effective in producing 5-year survival 2% of the time,19 health authorities in Ireland issued a warning advising cancer patients to seek the advice of their doctors or pharmacists before running to get GcMAF.20 Once again, the health authorities do their citizens a great disservice.
The fact is, 70% of cancers are solid tumors that are not penetrated by chemotherapy or radiation.21 GcMAF may be a cancer patient’s only real hope, saving 77% of terminal stage-4 tumor patients in clinics and 60% at home.
GcMAF around the Globe
People in 80 nations have used GcMAF. News stories about GcMAF are not published by newspapers or TV, but circulated via the Internet.
Conspiracy Theories Become Real
The GcMAF story gives every cancer conspiracy theorist all the evidence needed to convince others there is a concerted effort to hide what appears to be a bona-fide, natural cancer cure that can be spun out of blood plasma obtained from healthy adults. In this Internet era, how long can valid cancer cures be covered up? How many doctors can they shoot or otherwise kill (63 to date, a new one very week) before the public catches on?
Cancer Cells Activate Nagalase
Many natural medicine doctors in the U.S., who oppose mass childhood vaccination for infectious diseases, also recommend or administer GcMAF for cancer and other maladies. Moreover, some of these doctors accomplish full recovery of 60% of non-verbal autistic children and were said in news reports to have discovered that vaccines are laced with an enzyme, nagalase (N-acetyl-galactosaminidase) that facilitates the spread of cancer. GcMAF drastically reduces nagalase levels, and treats many vaccine-induced diseases.22
Strikingly, there is scientific evidence that vaccines do in fact provoke internal activation of nagalase.23 However, this fact may be exaggerated and misunderstood.
For vaccines to be effective the surrounding tissue must be broken down to facilitate penetration of the vaccine. Nagalase is an extracellular degrading enzyme that is secreted by cancerous cells in the process of tumor spread (metastasis) and invasion. Nagalase is a known marker of inflammation and high nagalase activity levels predict dire outcomes for cancer patients, and those with other diseases.24
However, should nagalase be found in vaccines or provoked by vaccination, this would only temporarily break down connective tissues. Cancer as a result of vaccination at the site of needle injection is not reported. However, there are reports of dogs developing cancer at the site of their vaccination needle injections at the nape of their neck.25
Understanding Non-Macrophage Mechanisms of GcMAF
There is a more compelling explanation for the profound cancer-quelling properties of GcMAF that go beyond its ability to activate cancer-engulfing, white-blood cells known as macrophages.
To understand this mechanism we need to shift from investigation of cancer cells to the biological real estate that surrounds them. Connective tissue or the “goo” that surrounds living cells must remain intact to maintain normal rates of cell growth and renewal. A biological phenomenon known as contact inhibition comes into play. Take a lab dish and instill some living cells into its center and the cells will grow to the edge of the dish and cease replication. Signals between cells that are in contact with each other control growth of new cells.
Disrupt the connective tissue and a growth signal emanates. This is a primary cause of cancer that has been demonstrated in the laboratory.
It was researcher Mary Helen Barcellos-Hoff who in 2011 demonstrated why the integrity of the gooey matrix that surrounds living cells (called connective tissue) is critically important in the prevention of cancer. In a landmark experiment, Barcellos-Hoff removed breast cells from their surrounding environment of hyaluronic acid-rich connective tissue and subjected that tissue to intense ionizing radiation, a known carcinogen. Then, the breast cells were returned to their environment, which experienced accelerated development of aggressive tumors even though they were never subjected to radiation.26
Connective tissue contains a water-holding Jello-like molecule called hyaluronic acid (HA). Chondroitin sulfate is a precursor for hyaluronan (hyaluronic acid).27 Decreased HA production leads to cancer progression.28
Hyaluronic acid (HA) is often mischaracterized as a facilitator for the spread of cancer (metastasis).29 A lab experiment dispelled the misconception that externally instilled HA may facilitate the spread of cancer.30 To be more accurate, degraded (fragmented) HA allows cancer cells to migrate and tumors to spread.31
Fragmented HA is required for cancer cells to escape interception by the immune system and invade distant tissues and organs.32
Cancer-proof Animals
Of considerable interest is the known fact that naked (hairless) mole rats that live ten-times longer than other rodents and almost never develop cancer because of their abundant production of hyaluronan (HA).33 It is the decreased activity of HA-degrading enzymes (hyaluronidase, nagalase) that results in the unusual cancer resistance observed in naked mole rats.34 To learn how naked mole rats do this naturally, you are urged to read my new book on the topic, which shall be released shortly through NHF.
It is no surprise to learn, then, that GcMAF abolishes cancer not only by activation of the human immune system and stimulation of macrophages that literally digest cancer cells, but by virtue of its ability to inhibit nagalase, an enzyme that degrades connective tissue surrounding tumors. When the integrity of connective tissue is maintained or restored, cancer cells cannot migrate; so, GcMAF stops the spread of cancer (metastasis) and cancer cells die in place.
This explains why most efforts to kill cancer cells are fruitless. Killing cancer cells doesn’t deal with the origin of the disease, which is loss of integrity of the connective tissue.
In regard to what readers have just learned about GcMAF and its ability to inhibit nagalase and maintain the integrity of connective tissue, it would by definition be less effective in non-solid tumors such as cancer of the blood (leukemia) and cancer of the lymph (lymphoma). However, this is where increased macrophage activity would counter the growth of cancer in non-solid tissues.
Mankind on the Cusp
Mankind is on the cusp of putting the most powerful yet non-toxic weapon into practice against cancer and many other maladies. But the medical establishment stands in its way.
Aside from recent developments involving GcMAF, a major change is already underway to abandon the slash-burn-poison approach to cancer therapy. An article entitled “Cancer Immunotherapy: The Beginning of the End of Cancer” says “the tide has finally changed and immunotherapy has become a clinically validated treatment for cancers.”35 Immunologists have removed T-cells from cancer patients, increased their numbers and activated them, then re-infused them to produce full cures for leukemia (cancer of the blood).36
Pharmaceutical companies now seek to acquire any developmental drug companies that have proven immunotherapies for cancer.37
So Why is There Such Opposition to GcMAF Therapy?
It has become obvious that agencies that approve cancer therapies have become guardians of the status quo. Cancer treatment centers are so steeped in debt (Sloan Kettering Cancer Center in New York holds $1.9 billion of debt), they cannot afford to cure cancer, especially with a simple and more economical therapy like GcMAF.
It is estimated that 80% of private practice oncologists’ income is generated from administration of ineffective intravenous administration of toxic chemotherapy.38 GcMAF would make cab drivers out of most oncologists.
A cure for cancer could vastly reduce the $4.95 billion a year spent on research doled out by the National Institutes of Health. The medical establishment is making a killing in a hollow effort to cure cancer. Medical costs to treat cancer now exceed $125 billion a year.
It would take many years and billions of dollars to prove GcMAF cures each and every form of cancer and gain approval from regulatory agencies.
GcMAF must be seen in light of toxic, ineffective but approved cancer treatments. As previously cited, chemotherapy contributes to the 5-year survival of cancer patients only 2% of the time. GcMAF therapy doesn’t have to do much to best existing cancer treatments. Furthermore, many cancer patients haven’t the time to wait for future cures.
It is interesting to note that insulin, penicillin, aspirin, and digitalis all came into common use without controlled studies. In fact, the standard blinded study where half the patients are given an active drug and the other half an ineffective placebo pill and neither patient or doctor know which pill was administered is not ethically acceptable in cancer therapy as half of the tested subjects would be left to die.
Since GcMAF is made in the human body, it is non-toxic. Very few GcMAF-treated patients experience a transient fever as a sluggish immune system is re-awakened. That is about all.
About 3% of blood plasma is Gc protein. Just a tiny fraction of that Gc protein, a billionth of a gram, is converted to GcMAF. About 2 million units of blood plasma are infused annually in the U.S. Via natural internal conversion of Gc protein to GcMAF, patients who receive infusions of blood plasma have indirectly been getting a mild form of GcMAF therapy.
Human safety studies using GcMAF have already been done. The Immuno Biotech GcMAF has been shown to inhibit blood-serum levels of nagalase in cancer patients without side effects.39 Until the UK’s Medicines & Healthcare products Regulatory Agency (MHRA) shut it down, Immuno Biotech’s GOLEIC version of GcMAF was available for worldwide shipment from its European source.40
In 2016, an estimated 1,685,210 new cases of cancer will be diagnosed and 595,690 Americans will die from the disease.41 These statistics are unacceptable in light of low-cost treatments such GcMAF that are available. While GcMAF safety studies have been published, a healthy person produces their own GcMAF without side effect, making concerns over safety moot.
Calculating a six-month course of GcMAF at a cost of $9600, would cost approximately $5.7 billion for all patients with terminal cancer. GcMAF treatment for all newly diagnosed cases of cancer at $9600 per patient would run about $16 billion, far short of the $125 billion now spent on largely ineffective and toxic cancer therapies.
Immuno Biotech claims it could drop the cost from $500 to $50 a vial with newer technology they have developed. It is the legal costs borne from 32 prosecutions from the MHRA, including 14 raids with 100 police, and 5 arrests, that have thwarted the availability of even better GcMAF including oral, non-injectable product.
This health writer personally observed a 53-year old man with terminal stage-4 pancreatic cancer (the same type of cancer that afflicted the late Steve Jobs of Apple Computers) who used Immuno Biotech’s GOLEIC. In just three months that patient’s cancer markers were normalized, he has been taken off chemotherapy, and is remarkably healthy. Seeing GcMAF work first hand is very convincing.
If modern medicine chooses not embrace GcMAF therapy, it appears cancer patients, at least a segment of them who have undergone unsuccessful treatment and have no other options, may choose to bolt and opt for GcMAF therapy. This is especially true since treatment can be accomplished safely at home without medical supervision.
Cancer researchers will continue to say: “the war on cancer may never be won.” But the cancer research community keeps pocketing billions of dollars for a cure they say is “impossible.”42 The cancer industry has turned out to be a giant jobs program where over a half-million Americans must die every year to ensure hundreds of thousands of jobs for doctors, nurses, pharmacists, researchers, pharmaceutical-industry employees, and government regulators.
Health regulators will continue to say GcMAF therapy is unproven. Yes, but it is not disproven. It just doesn’t have an official stamp of approval on it. And it works every day to prevent cancer in our healthy bodies.
We have evidence now from human use with a few thousand patients and corroborative evidence from an animal model, that cancer can be cured in the full sense of the word. Immuno Biotech is light years ahead of the National Cancer Institute in its GcMAF knowledge base. Furthermore, safety studies have been published.
With so many lives at risk and no real cures at hand, the Food and Drug Administration would be wise to allow GcMAF therapy to proceed with the requirement of real-time adverse event reporting to ensure patient safety. Since it is safe, let GcMAF move to the clinic where oncologists can report on its effectiveness in progress, just as when insulin, penicillin, and aspirin were put into common use.
This is the cure for cancer.
It is now biologically possible humans can live completely immune from cancer during their lifetime. The problem is that current cancer research and the treatment industry has to step aside for that to happen.
How much longer, in this era of open information, can the controlling authorities stonewall the public about GcMAF therapy and keep it hidden in the closet?
________________________
- Ruth Evans, “Investigation over cancer ‘cure’ GcMAF in health food shop,” BBC News, October 16, 2016, at http://www.bbc.com/news/health-37654666.
- Aine McCarthy, “News digest – GcMAF, hijacking immune cells, manipulation of blood vessels and… nose-to-tail eating?,” Cancer Research UK, October 22, 2016, at http://scienceblog.cancerresearchuk.org/2016/10/22/news-digest-gcmaf-hijacking-immune-cells-manipulation-of-blood-vessels-and-nose-to-tail-eating/.
- Supra, BBC News.
- WorldWideScience.org, at https://worldwidescience.org/topicpages/m/macrophage-activating+factor+gcmaf.html.
- See https://www.thenhf.com/hfn-magazine/health-freedom-articles/125-2010-articles/2327-29cancer-cured-for-good.
- Gregory KJ, Zhao B, et al., “Vitamin D Binding Protein-Macrophage Activating Factor Directly Inhibits Proliferation, Migration, and uPAR Expression of Prostate Cancer Cells,” PLOS, October 18, 2010, at http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0013428.
- EFRANAT, Research & Development, at http://efranat.com/Research-Development1/Canines-Treatment-Results.aspx.
- Bill Sardi, “Is Cancer Being Cured Right Before Our Eyes? Cancer Center In Japan Reports Startling Remissions Using Vitamin & Immunotherapy Regimen,” LewRockwell.com, June 22, 2013, at https://www.lewrockwell.com/2013/06/bill-sardi/is-cancer-being-cured/.
- See http://www.saisei-mirai.or.jp/gan/macrophage_research_eng.html. See also Inui T, Amitani H, et al., “Case Report: A Non-small Cell Lung Cancer Patient Treated with GcMAF, Sonodynamic Therapy and Tumor Treating Fields,” Anticancer Res., 2016 Jul;36(7):3767-70, at https://www.ncbi.nlm.nih.gov/pubmed/27354652; Inui T, Makita K, “Case report: A breast cancer patient treated with GcMAF, sonodynamic therapy and hormone therapy,” Anticancer Res., 2014 Aug;34(8):4589-93, at https://www.ncbi.nlm.nih.gov/pubmed/25075104.
- Inui T, Kuchiike D, et al., “Clinical experience of integrative cancer immunotherapy with GcMAF,” Anticancer Res., 2013 Jul;33(7):2917-9, at https://www.ncbi.nlm.nih.gov/pubmed/23780980.
- Ruggiero M, Ward E, et al., “Oleic Acid, Deglycosylated Vitamin D-Binding Protein, Nitric Oxide: A Molecular Triad Made Lethal to Cancer,” Anticancer Res., 34: 3569-3578 (2014), at http://immunocentre.eu/wp-content/uploads/2014/09/AnticancerResearchClinic.pdf.
- See, e.g., https://gcmaf.se/videos/.
- See https://gcmaf.se/wp-content/uploads/2013/06/Gpressnigel.pdf.
- Isobel Dickinson, “Miracle mum: ‘Vampire’ cancer treatment saved my life after I was given months to live,” Daily Star Sunday, April 17, 2016, at http://www.dailystar.co.uk/news/latest-news/508667/gran-Gail-Harling-cured-of-cancer-by-vampire-blood-injection-replacement-treatment.
- Staff writer, “Drug banned in Guernsey amid fears it’s not fit for consumption,” ITV, last updated May 7, 2015, at http://www.itv.com/news/channel/update/2015-02-16/hssd-we-are-looking-for-alternatives-to-gcmaf/.
- Kanda S, Mochizuki Y, et al., “Effects of Vitamin D3-Binding Protein-Derived Macrophage Activating Factor (GcMAF) on Angiogenesis,” J Natl Cancer Inst, (2002) 94 (17): 1311-1319; DOI: https://doi.org/10.1093/jnci/94.17.1311, at https://academic.oup.com/jnci/article/94/17/1311/2519874/Effects-of-Vitamin-D3-Binding-Protein-Derived.
- Staff writer, “Guernsey’s Chief Pharmacist says he has a duty to the public over GcMAF,” ITV, Feb 6, 2017, at http://www.itv.com/news/channel/update/2015-02-06/guernseys-chief-pharmacist-says-he-has-a-duty-to-the-public-over-gcmaf/.
- Staff writer, “Drug banned in Guernsey amid fears it’s not fit for consumption,” ITV, last updated May 7, 2015, at http://www.itv.com/news/channel/update/2015-02-16/hssd-we-are-looking-for-alternatives-to-gcmaf/.
- Morgan G, Ward R, Barton M, “The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies,” See comment in PubMed Commons belowClin Oncol (R Coll Radiol), 2004 Dec;16(8):549-60, at https://www.ncbi.nlm.nih.gov/pubmed/15630849.
- Joe Leogue, “Cancer patients warned to avoid unauthorised ‘miracle cure’,” Irish Examiner, Jan 19, 2016, at http://www.irishexaminer.com/ireland/cancer-patients-warned-to-avoid-unauthorised-miracle-cure-376862.html.
- Tannock IF, Lee CM, et al., “Limited Penetration of Anticancer Drugs through Tumor Tissue,” Clinical Cancer Research, Vol 8, Issue 3 (March 2002), at http://clincancerres.aacrjournals.org/content/8/3/878.full.
- John P. Thomas, “Is The U.S. Medical Mafia Murdering Alternative Health Doctors Who Have Real Cures Not Approved by the FDA?,” Health Impact News, accessed May 12, 2017, at https://healthimpactnews.com/2015/is-the-u-s-medical-mafia-murdering-alternative-health-doctors-who-have-real-cures-not-approved-by-the-fda/.
- See Chemical & Pharmaceutical Bulletin Feb 1991, at https://www.jstage.jst.go.jp/article/cpb1958/39/2/39_2_505/_pdf.
- Hartmann D, Von Figura G, et al., “Plasma N-acetyl-glucosaminidase in advanced gastro-intestinal adenocarcinoma correlates with age, stage and outcome,” Future Oncol, 2015;11(2):193-203, at https://www.ncbi.nlm.nih.gov/pubmed/25040106.
- Pet Vaccinations Induce Cancerous Growths,” VacTruth, undated, at https://vactruth.com/2012/05/23/pet-vaccinations-cancer/.
- Nguyen DH, Oketch-Rabbah HA, et al., “Radiation Acts on the Microenvironment to Affect Breast Carcinogenesis by Distinct Mechanisms that Decrease Cancer Latency and Affect Tumor Type,” Cancer Cell, Vol 19, Issue 5, p 640-651, 17 May 2011, at http://www.cell.com/cancer-cell/abstract/S1535-6108(11)00121-8.
- Czoka AB & Stern R, “Hypotheses on the evolution of hyaluronan: a highly ironic acid,” Glycobiology, 2013 Apr;23(4):398-411. doi: 10.1093/glycob/cws218. Epub 2013 Jan 12, at https://www.ncbi.nlm.nih.gov/pubmed/23315448.
- Poukka M, Bykachev A, et al., “Decreased expression of hyaluronan synthase 1 and 2 associates with poor prognosis in cutaneous melanoma,” BMC Cancer, 2016 May 16;16:313. doi: 10.1186/s12885-016-2344-8, at https://www.ncbi.nlm.nih.gov/pubmed/27184066.
- Venning FA, Wullkopf L & Erler JT, “Targeting ECM Disrupts Cancer Progression,” Front Oncol, 2015 Oct 20;5:224. doi: 10.3389/fonc.2015.00224. eCollection 2015, at https://www.ncbi.nlm.nih.gov/pubmed/26539408.
- Seino S, et al., “No influence of exogenous hyaluronan on the behavior of human cancer cells or endothelial cell capillary information,” Journal Food Science, July 2014.
- Monslow J, Govindaraju P & Puré E, “Hyaluronan – a functional and structural sweet spot in the tissue microenvironment,” Front Immunol, 2015 May 15;6:231, doi: 10.3389/fimmu.2015.00231. eCollection 2015, at https://www.ncbi.nlm.nih.gov/pubmed/26029216; Wu RL, Huang L, et al., “Hyaluronic acid in digestive cancers,” J Cancer Res Clin Oncol, 2017 Jan;143(1):1-16. doi: 10.1007/s00432-016-2213-5, Epub 2016 Aug 17, at https://www.ncbi.nlm.nih.gov/pubmed/27535565; Tsepilov RN & Beloded AV, “Hyaluronic Acid–an “Old” Molecule with “New” Functions: Biosynthesis and Depolymerization of Hyaluronic Acid in Bacteria and Vertebrate Tissues Including during Carcinogenesis,” Biochemistry (Mosc), 2015 Sep;80(9):1093-108. doi: 10.1134/S0006297915090011, at https://www.ncbi.nlm.nih.gov/pubmed/26555463.
- Turley EA, Wood DK, et al., “Carcinoma Cell Hyaluronan as a “Portable” Cancerized Prometastatic Microenvironment,” See comment in PubMed Commons belowCancer Res, 2016 May 1;76(9):2507-12. doi: 10.1158/0008-5472.CAN-15-3114. Epub 2016 Apr 20, at https://www.ncbi.nlm.nih.gov/pubmed/27197262.
- Fisher GJ, “Cancer resistance, high molecular weight hyaluronic acid, and longevity,” See comment in PubMed Commons belowJ Cell Commun Signal, 2015 Mar;9(1):91-2. doi: 10.1007/s12079-015-0278-6. Epub 2015 Mar 5, at https://www.ncbi.nlm.nih.gov/pubmed/25740467.
- Tian X, Azpurua J, et al., “High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat,” Nature, 2013 Jul 18;499(7458):346-9. doi: 10.1038/nature12234. Epub 2013 Jun 19, at https://www.ncbi.nlm.nih.gov/pubmed/23783513.
- Farkona S, Diamandis EP & Blasutig IM, “Cancer immunotherapy: the beginning of the end of cancer?,” BMC Med, 2016 May 5;14:73. doi: 10.1186/s12916-016-0623-5, at https://www.ncbi.nlm.nih.gov/pubmed/27151159.
- Stephen Matthews, “Revolutionary cancer treatment hailed as a ‘game changer’ could be made more effective for millions of sufferers, scientists say,” Daily Mail, Nov 8, 2016, at: http://www.dailymail.co.uk/health/article-3917434/Revolutionary-cancer-treatment-hailed-game-changer-effective.html#ixzz4gqWblp4r Bolli E, Movahedi K, et al., “Novel insights in the regulation and function of macrophages in the tumor microenvironment,” Curr Opin Oncol, 2017 Jan;29(1):55-61, at https://www.ncbi.nlm.nih.gov/pubmed/27792052.
- Harris SJ, Brown J, et al., “Immuno-oncology combinations: raising the tail of the survival curve,” Cancer Biol Med., 2016 Jun;13(2):171-93. doi: 10.20892/j.issn.2095-3941.2016.0015, at https://www.ncbi.nlm.nih.gov/pubmed/27458526.
- Integrated Healthcare Strategies, Medical Oncology Compensation Survey Report 2013, at http://www.integratedhealthcarestrategies.com/documents/news/77c210ca-4613-4f00-a2e2-b117cb5ff1d5.pdf.
- Thyer L, Ward E, et al., “GC protein-derived macrophage-activating factor decreases α-N-acetylgalactosaminidase levels in advanced cancer patients,” Oncoimmunology, 2013 Aug 1; 2(8): e25769; Published online 2013 Jul 29. doi: 10.4161/onci.25769, at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3812199/.
- See https://gcmaf.se/.
- National Cancer Institute, “Cancer Statistics,” last updated March 22, 2017, at https://www.cancer.gov/about-cancer/understanding/statistics.
- Lizzie Parry, “’The war on cancer may NEVER be won’: Cure ‘could be impossible’ because the disease is so highly evolved,” Daily Mail, August 22, 2014, at http://www.dailymail.co.uk/health/article-2731765/The-war-cancer-NEVER-won-Cure-impossible-disease-highly-evolved.html#ixzz4gqMjQtHN.
